Medicaid innovation pathway: How 1115 waivers work
Medicaid leaders face a long runway when trying to improve their programs and meet state and territory goals for health and wellness.
Medicaid programs understand that 1115 waivers are one of the few ways to innovate to meet state- and territory-identified goals for health and wellness of Medicaid members. In this resource, NAMD digs into these waivers to help policymakers and partners understand the steps in this often lengthy, complex process for approval of a new 1115 waiver and the timeframe for taking a policy from concept to impact.
What are the rules governing 1115 waivers?
Federal rules set minimum standards for states and territories related to who qualifies for Medicaid and the benefits that must be provided. Section 1115 of the Social Security Act gives the Secretary of Health and Human Services (HHS Secretary) authority to allow states and territories to waive federal rules to carry out demonstration projects that will promote the objectives of the Medicaid program. This broad authority allows states and territories flexibility to design and improve their Medicaid programs and can be authorized across the entire program or more narrowly focused on a specific population of Medicaid members. These waivers are governed by two operating principles.
- 1115 demonstrations must be budget neutral. No 1115 demonstration can cost the federal government additional money, meaning the proposed federal Medicaid expenditures cannot be more than anticipated federal spending without the demonstration. The HHS Secretary can permit federal financial participation (FFP) funding for costs not otherwise matchable under federal rules, like temporary housing for individuals with serious mental illness, using anticipated savings gained through the health benefits of stable housing to the individuals covered. However, budget neutrality is not defined in statute or rule and definitions are subject to CMS agency policy, which has changed over time.
- 1115 demonstrations are ultimately granted at the discretion of the HHS Secretary. Demonstration waivers are subject to a great deal of discretion by the HHS Secretary, so states and territories seeking Section 1115 waivers must engage in often lengthy negotiation processes with CMS. CMS performs a case-by-case review of each policy within a proposed demonstration to determine whether the objectives align with Medicaid and federal policy. This review and the subsequent negotiations between the requesting state or territory and the federal government can take months and sometimes years. Once approved, 1115 waivers are initially granted for five years.
For additional background information see MACPAC overview of Section 1115 waivers and CMS overview of Section 1115 waivers.
How does the process work?
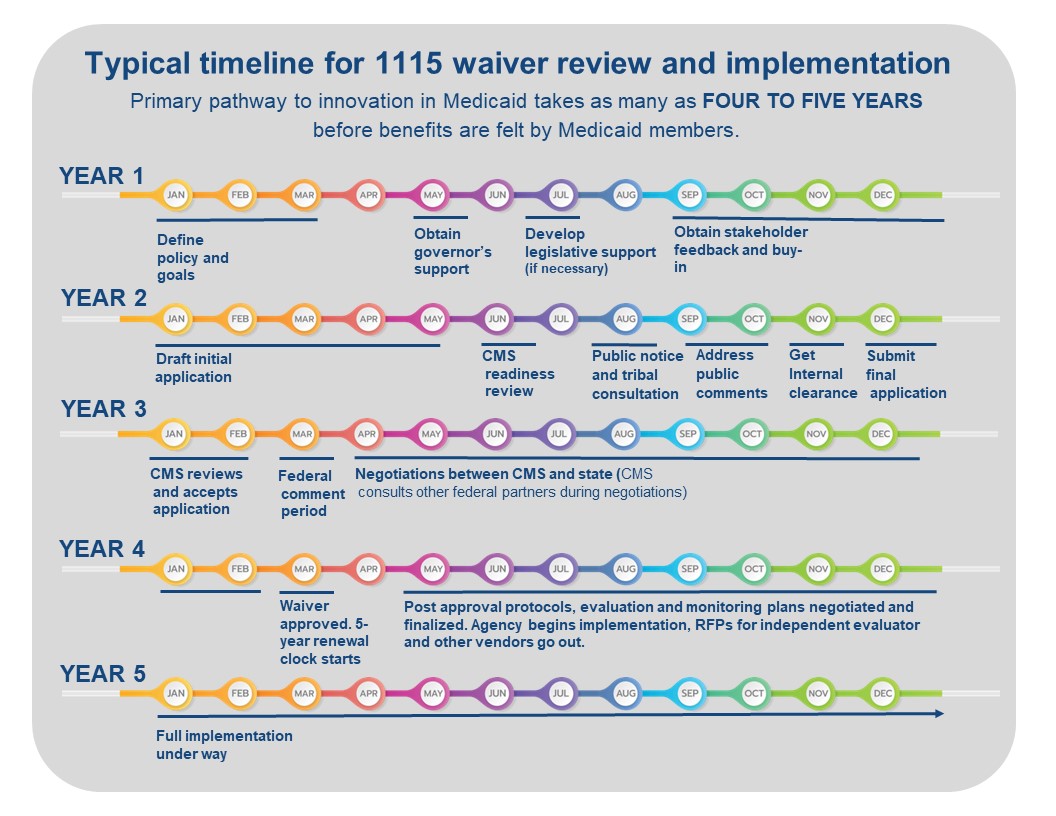
Step 1: Defining the policy and goals
Typical timeframe: 6 months to 1 year
Initial policy contemplation involves defining one or more changes in a state or territory’s Medicaid program that will improve the overall program and experience for Medicaid members but cannot be accomplished through a State Plan Amendment (SPA) or change in state rules or statute. The proposed policy may also include a request for federal financial participation (FFP) for costs not otherwise matchable using budget neutrality savings. After initial policy development, states or territories then often test interest with stakeholders including providers, managed care organizations, Medicaid members and their advocates. States and territories will consider involvement by legislators and potential legislation with associated political timelines at this stage. Buy-in at the governor’s office is critical in the process as the waiver application must include support from the governor. Additionally, states or territories may float initial concepts by CMS by submitting ideas in white papers.
Step 2: Draft 1115 waiver application
Typical timeframe: 6 months to 1 year
Once proposed policies have been developed, a formal waiver demonstration application with requirements for monitoring and evaluation must be drafted. A draft 1115 waiver application will include the hypotheses for outcomes of proposed waiver policies, evidence-based arguments with supporting data for proposed new policies, demonstration that policies cannot be achieved through other less complicated mechanisms like a SPA, statement of the federal authorities to waive, number of affected beneficiaries, an evaluation plan, budget neutrality calculations, any costs otherwise unmatchable requested, public notice timeline and announcements including formal written notification of consultation to tribal leaders of the federally recognized tribes in in the requesting state or territory. A state or territory may set up a meeting with CMS and/or HHS leadership to present policies and intent as an initial test of their planning.
Step 3: State/territory notice and comment period
Typical timeframe: 2 to 3 months
Once a draft 1115 demonstration is developed, CMS will review the application for completion and provide feedback. Once the initial CMS review for completeness is done, there is a 30-day official public comment period, which all states and territories must observe, with time after needed to address all public comments. Strict policies around the public notice and tribal consultation exist, and if not followed a state or territory will be required to re-do the public notice process from the beginning, which can create significant and unnecessary delays.
Step 4: Final application submitted to CMS
Typical timeframe: 1 to 2 months
The final application that is sent to CMS must include appendices with responses to all public comments and public notice documentation from Step 3. Additionally, the final application must include a cover letter from the governor, which can require extra time for clearance and review depending on state practice.
Step 5: Federal notice and comment period
Typical timeframe: 1 to 3 months
CMS conducts a completeness review of the application followed by a 30-day federal public comment period. CMS must respond to public comments on the waiver. The volume and nature of public comments received, as well as how many other waiver applications CMS is concurrently considering, can impact how long this step takes. During this step, CMS may also meet with advocates or other stakeholders who provide public comment on the state or territory’s waiver.
Step 6: Federal and state/territory negotiations
Typical timeframe: 6 months to 2 years
This is the most complex and lengthy phase in the waiver process. Each policy in a waiver must be negotiated separately. During the initial negotiation phase, CMS may provide questions and requests for additional information or clarification on policies or data contained in the application. This can result in months of back and forth between a state or territory and CMS on each policy, with lots of requests for various iterations of information. The negotiation process requires state and territory staff dedicated to providing timely responses to CMS’s additional requests to ensure the negotiations keep moving forward. CMS confers with other federal partners during this phase as well, which may increase the time it takes to get approval. This typically includes:
- The White House Office of Management and Budget, which plays a key role in negotiations on the budget neutrality component of the waiver; and
- Other HHS agencies, depending on the policy areas included in the waiver, such as the Substance Abuse and Mental Health Services Administration, the Health Resources and Services Administration, and the Administration for Community Living.
To help keep the process moving, a state or territory may request to have regular meetings with CMS to provide information and further discuss policies and data. Policies that have already been approved in another state can often be implemented more quickly than novel policies. Once CMS has gained internal approval from the HHS Secretary, waiver special terms and conditions are drafted and sent to the state or territory. Finally, the state or territory comments on the terms and conditions and negotiates with CMS until details of the waiver agreement are finalized.
Step 7: Federal approval
Typical timeframe: 1 month
At approval of the final terms and conditions, CMS sends the final approval with a cover letter addressed to the Medicaid Director. The cover letter may acknowledge requested policies that were not approved or are still in discussion. HHS and/or CMS and the state or territory may issue press releases at the time of approval requiring coordination and clearance.
Step 8: Pre-implementation
Typical timeframe: 3 months to 1 year
The wavier cover letter will highlight topics, not included in the final terms and conditions, that will be negotiated between the state or territory and CMS in a post-approval period. Post-approval agreements are finalized within a time period defined in the cover letter and eventually included in the waiver appendices. These appendices include documents like the final evaluation and monitoring plans, protocols for drawing down federal financial participation dollars if applicable, and implementation plans.
Step 9: Waiver implementation
Typical timeframe: 5 years
States and territories are required to host post-waiver approval public forums within 6 months of approval. States and territories must procure an independent evaluator for the waiver and finalize an evaluation plan. Finally, states and territories begin incorporating the new policy into Medicaid operations, which will be monitored and evaluated per the plans agreed to by the state or territory and CMS. Depending on the complexity of the policy change that is being implemented, it may take anywhere from 1 to 3 years after waiver approval for Medicaid members to begin to experience the impact of the policy change.
Policymakers and advocates can best partner with Medicaid by understanding the complexity of the 1115 waiver process
The 1115 waiver is a critical tool for Medicaid innovation on behalf of Medicaid members and their health. But the timeline and process to leverage this innovation pathway is incredibly complex and takes significant time and Medicaid agency investment of resources and staffing to accomplish. Policymakers and advocates can help Medicaid programs advance their state or territory’s innovation goals by understanding the elements of this process and what is required to get from policy concept to implementation.
Related resources
NAMD Requests the Centers for Medicare & Medicaid Services Revisit their Section 1115 Waiver Rebasing Policies
NAMD Calls for Swift Action from the Center for Medicaid and CHIP Services and the Office of Management and Budget to Ensure Provider Solvency
Stay Informed
Drop us your email and we’ll keep you up-to-date on Medicaid issues.